Structure Determination of Biomolecules
What is NMR?
Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical chemistry technique used in quality control and research for determining the content and purity of a sample as well as its molecular structure.
For example, NMR can quantitatively analyze mixtures containing known compounds. For unknown compounds, NMR can either be used to match against spectral libraries or to infer the basic structure directly. Once the basic structure is known, NMR can be used to determine molecular conformation in solution as well as studying physical properties at the molecular level such as conformational exchange, phase changes, solubility, and diffusion. In order to achieve the desired results, a variety of NMR techniques are available.
Source: http://chem.ch.huji.ac.il/nmr/whatisnmr/whatisnmr.html#top
Why do we need NMR?
 The structure of a molecule strongly influences a substance’s physical and chemical properties and, as a result, how it reacts with other molecules or affects living organisms. For example, ethanol – also known as absolute alcohol – and dimethyl ether are composed of the same type and number of atoms; two carbon, six hydrogen and one oxygen. However, the structures, and therefore the properties, are quite different. Ethanol is a liquid, while dimethyl ether is a poisonous gas!
The structure of a molecule strongly influences a substance’s physical and chemical properties and, as a result, how it reacts with other molecules or affects living organisms. For example, ethanol – also known as absolute alcohol – and dimethyl ether are composed of the same type and number of atoms; two carbon, six hydrogen and one oxygen. However, the structures, and therefore the properties, are quite different. Ethanol is a liquid, while dimethyl ether is a poisonous gas!What is X-Ray Crystallography?
X-ray crystallography is a technique for determining the three-dimensional structure of molecules, including complex biological macromolecules such as proteins and nucleic acids. It is a powerful tool in the elucidation of the three-dimensional structure of a molecule at atomic resolution. Data is collected by diffracting X-rays from a single crystal, which has an ordered, regularly repeating arrangement of atoms. Based on the diffraction pattern obtained from X-ray scattering off the periodic assembly of molecules or atoms in the crystal, the electron density can be reconstructed.
Source: https://www.sciencedirect.com/science/article/pii/B0122270800013963
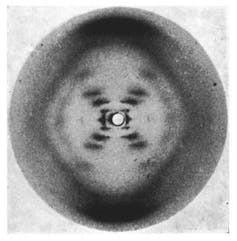
The famous photograph 51. Raymond Gosling/King’s College London
Today we know that inside crystals there are atoms arranged in regular patterns. Each of these atoms has a dense core containing neutrons and protons, and less dense outer shells containing electrons. X-rays that hit these obstacles interact with other X-rays that cause a phenomenon called diffraction, something all waves (light or sound) undergo whenever they hit obstacles.
In 1953 Rosalind Franklin used X-ray crystallography to produce images from a crystal of DNA. One of those images, called photograph 51, eventually led James Watson, Francis Crick and Maurice Wilkins to describe the helical structure of DNA is dubbed the most important “photograph” ever taken.
Source: http://theconversation.com/explainer-what-is-x-ray-crystallography-22143
What is cryo-EM?
Transmission electron microscopes (TEMs) use a beam of electrons to examine the structures of molecules and materials at the atomic scale. As the beam passes through a very thin sample, it interacts with the molecules, which projects an image of the sample onto the detector (often a charge-couple device; CCD). Because the wavelength of electrons is much shorter than that of light, it can reveal much finer detail than even super-resolution light microscopy.
But some materials – particularly biomolecules – are not compatible with the high-vacuum conditions and intense electron beams used in traditional TEMs. The water that surrounds the molecules evaporates, and the high energy electrons burn and destroy the molecules. Cryo-EM uses frozen samples, gentler electron beams and sophisticated image processing to overcome these problems.
But can’t you look at proteins using x-ray diffraction or NMR?
X-ray diffraction can give very high resolution structures of biomolecules, and several Nobel prizes have been awarded for just that. But to get an x-ray structure, you need to be able to crystallise the molecule. Many proteins won’t crystallise at all. And in some cases, the process of crystallisation alters the structure, so it’s not representative of what the molecule looks like in real life.
Cryo-EM doesn’t require crystals, and it also enables scientists to see how biomolecules move and interact as they perform their functions, which is much more difficult using crystallography.
NMR can also give very detailed structures, and investigate conformational changes or binding of small molecules. But it’s limited to relatively small proteins or parts of proteins, and usually soluble intracellular proteins, rather than those embedded in cell membranes. If you want to study larger proteins, membrane-bound receptors, or complexes of several biomolecules together, cryo-EM is where it’s at.
Source: https://www.chemistryworld.com/news/explainer-what-is-cryo-electron-microscopy/3008091.article
